By Eleftheria Safarika
There are two models we use today, for describing an atom’s structure: the Bohr model, and the quantum mechanical one. The Bohr atomic model, otherwise known as Bohr-Rutherford model, or cake model, was proposed by the Danish physicist Niels Bohr in 1913, as a description of the structure of atoms, and especially of the hydrogen atom. This description was radical, and differed from previous approaches, in the sense that it included quantum theory.
In general, the Bohr model describes the atom as consisting of a positive charged nucleus, surrounded by electrons in defined circular orbits with discrete radii, a description which very much resembles our Solar System, with the nucleus playing the role of the sun, and the electrons the role of the planets . These orbits are symbolized by the capital letters K, L, M, N, O, P, Q, and each is labeled by a letter n, the quantum number, an integer ranging from 1 to 7. Electrons are placed in these orbits in a specific way, and each orbit can only hold a certain number of electrons, K taking up to 2, L up to 8, M up to 18, N up to 32, O up to 32, and P up to 18, and Q up to 8. Each orbit had to be filled, and then remaining electrons would be placed on a higher orbit. The energy of each orbit increases as we get farther and farther away from the nucleus. So electrons in farther orbits have more energy. The term we use for these orbits is energy levels, or otherwise shells, which aims to indicate this difference in the energy between two shells. The energy of an electron in any shell is what we call quantized, which means it can only take discrete values depending on its energy level, but can take no other value in-between the allowed ones of two energy levels.
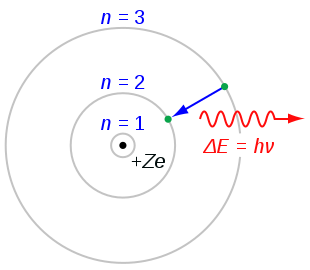
Electrons can “jump” between two orbits, between allowed, stationary states, but only by absorbing or emitting energy. The normal energy level of an electron, without it taking or releasing energy, is what we call a stable state, while moving to a higher, less stable orbit, is what we call an excited state. When the electron receives no more energy to allow it to remain in the excited state, it returns to its stable state, by releasing the extra energy. So, jumping to a higher orbit would require energy, while “immigrating” to a lower orbit would release energy. But in the second case, in what form would this remaining energy be released? Bohr suggested that the light emitted from hydrogen atoms, was the result of the transition of an electron from an orbit farther from the nucleus, to an orbit closer to the nucleus. This energy is released as a quantum of light, with the exact same energy the electron lost. For example, in this picture we see an electron moving from the orbit n=3 to n=2, therefore releasing energy. In hydrogen, an electron in an excited state in the orbit n=2, can fall back to its ground (stable) state, by releasing energy, as a photon of red light, in this case with a wavelength of 656 nm.
With this model, Bohr was able to explain the spectral series, the regular patterns of discrete wavelengths of the light emitted by hydrogen atoms. However, this model does not predict the relative intensities of the spectral lines. The Bohr model, in general, is a simple model, which is very useful in understanding the fundamentals of how atoms work, and this is why it’s so widely taught. It, also, explains properties of heavy atoms, which had never been explored and explained before. It answers the question why atoms got smaller from left to right, on the same period of the periodic table, even though more protons and electrons are gradually added. It also explains the behavior of the noble gases, and also why atoms on the left side of the periodic table lose electrons, while those on the right gain. There are some problems with the Bohr atomic model, such as it not corresponding to the Heisenberg Uncertainty Principle, but it remains important and radical in its way of presenting the atoms’ structure.
References
The Editors of Encyclopaedia Britannica, Bohr atomic model. Retrieved from: https://www.britannica.com/science/Bohr-atomic-model
Bohr model. Retrieved from: https://en.wikipedia.org/wiki/Bohr_model
Bohr’s Model of Atom. Retrieved from: https://www.toppr.com/guides/chemistry/structure-of-atom/bohrs-model-of-atom/